Hormone-independent luteinization as a physiological trigger of ovulatory gonadotropins surge in the human
The process of ovulation in the human is central not only to the field of infertility but to obstetrics and gynecology in general and has been a subject of thousands of research projects for almost a century. The holy grail of ovulation is the trigger mechanism for the surge of gonadotropins, LH and FSH, which according to the current paradigm is caused by sustained elevation of estradiol that culminates the end of the follicular phase.
Despite appearance that the role of estradiol in ovulation is a settled science, an in-depth look into the published data reveals considerable confusion about the relative roles of progesterone and estradiol in the ovulation process, which is acknowledged not only by peer-reviewed articles but also by textbooks.
This review provides compelling evidences that the role of estradiol in ovulation has been misinterpreted and the true physiological trigger of ovulation is pre-ovulatory progesterone surge in circulation to about 0.5 ng/ml. This, easy to miss, rise is hormone-independent, a reflection of the spontaneous luteinization of some granulosa cells within the follicle as it begins to lose integrity.
In addition to a synthesis of evidences placing progesterone as the most upstream messenger for GnRH ovulatory signaling pathway, current work reconciles progesterone’s ability to block ovulation most of the time, with its ability to trigger ovulation within a very narrow window of opportunity.
Accepting the role of progesterone as ovulation trigger, helps to explain such well known and puzzling phenomena as infertility linked to shorter follicular phase, shortening menstrual cycle with age, premature ovulation, improvement of infertility treatment outcomes with mild stimulation regiments, recovery of the post-mature oocytes and more.
Furthermore, this review discusses how the new ovulation paradigm may provide physicians with tools to improve ovulation induction and mysterious egg quality as well as perhaps even reduce the number of chromosomally abnormal conceptions.
Introduction
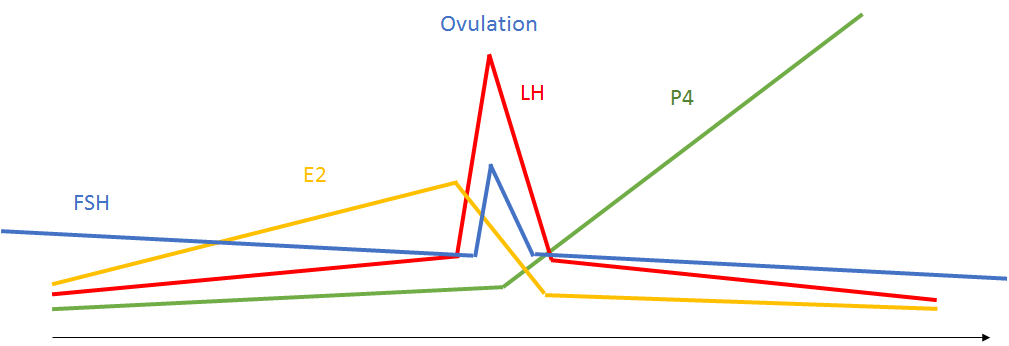
Figure 1. Current ovulation paradigm
LH independent luteinization, resumption of meiosis and follicle’s ruptur
Even though under physiological conditions LH is required for luteinization, follicle rupture and resumption of meiosis, understanding of ovulation requires appreciation of the fact that all ovulation events can take place without the LH.
LH independent luteinization
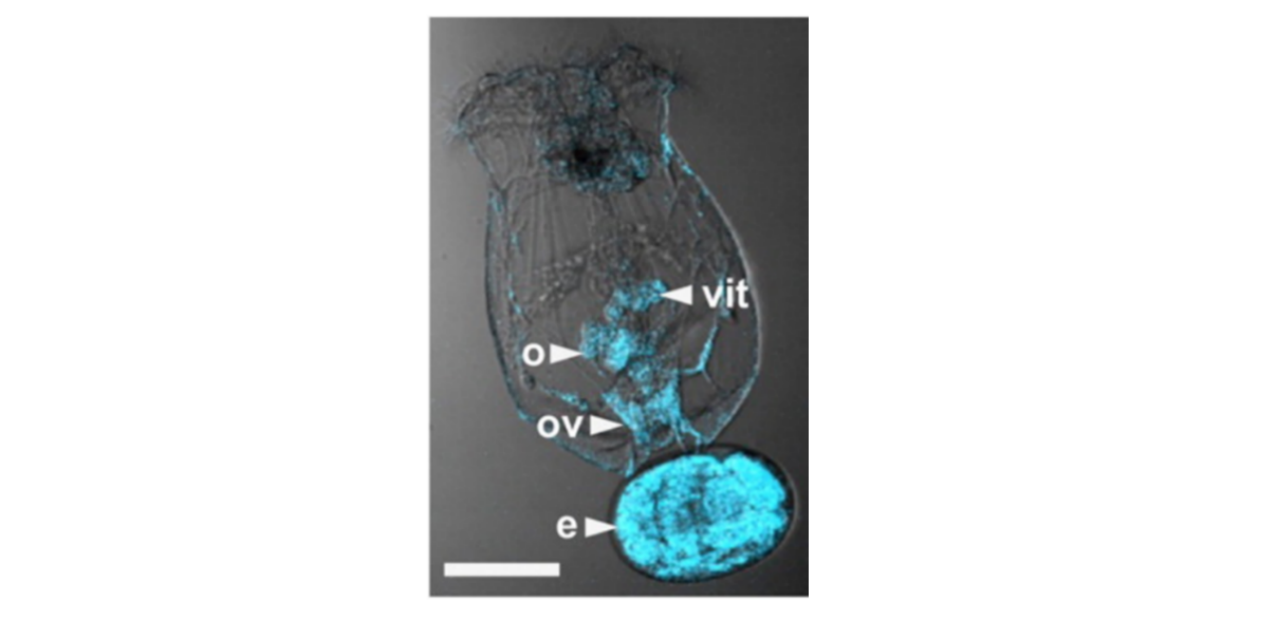
Figure 2. From Stout et all (2007). Note that cells surrounding the egg (e)of Brachionus Manjavacas are stained by antibodies to the progesterone (blue).
Luteinization is a default differentiation of all granulosa cells.
In vivo, the granulosa cells are committed to luteinization, but are prevented from the luteinization through the mechanism that is not fully understood and beyond the scope of this review. However, irrespective of the details, the maintenance of the block requires an intact base membrane of the follicle. Once base membrane is disrupted, whether through inflammation, or activated collagenase, or by intrafollicular pressure (Takahashi and Ohnishi, 1995), the granulosa cells are released from the differentiation block and become luteinized (Tedeschi et all, 1992).
Interestingly, the pace of luteinization of the intact follicle removed from the ovary is the same in the presence or absence of LH. This strongly supports the assertion that the release from the block is due to the changes in intrafollicular milieu rather than an induction of luteinization by LH (Wehrenberg and Rune, 2000).
Consistently with above, in hypophysectomized rats, completely lacking LH, follicles undergo normal ovulation and luteinization following injection of FSH (Hubbard and Erickson, 1988).
LH independent meiosis resumption
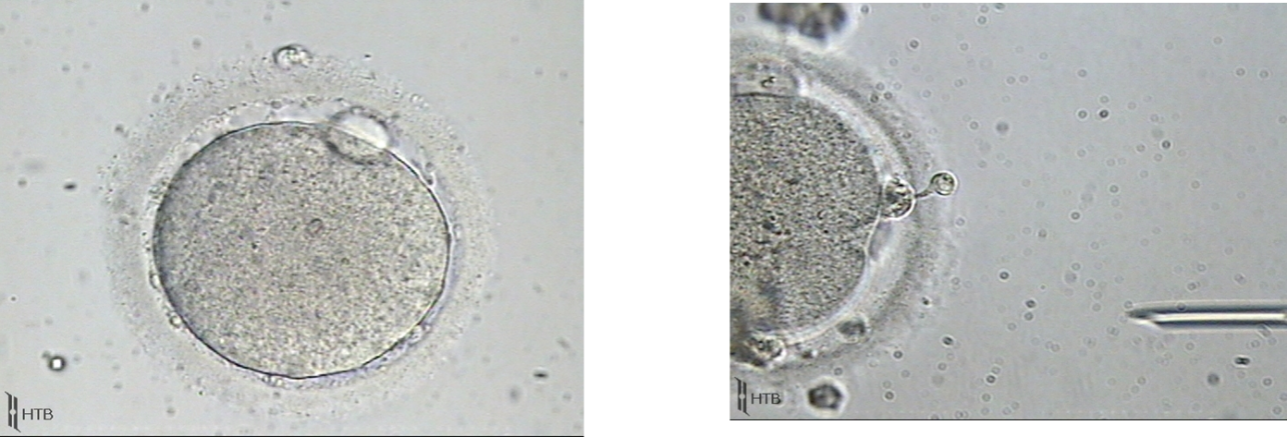
Figure 3. Debris from the degenerated gap-junctions and corona cell pulled under the zona pellucida due to the failure of its gap junctions degeneration. (Dozortsev, unpublished)
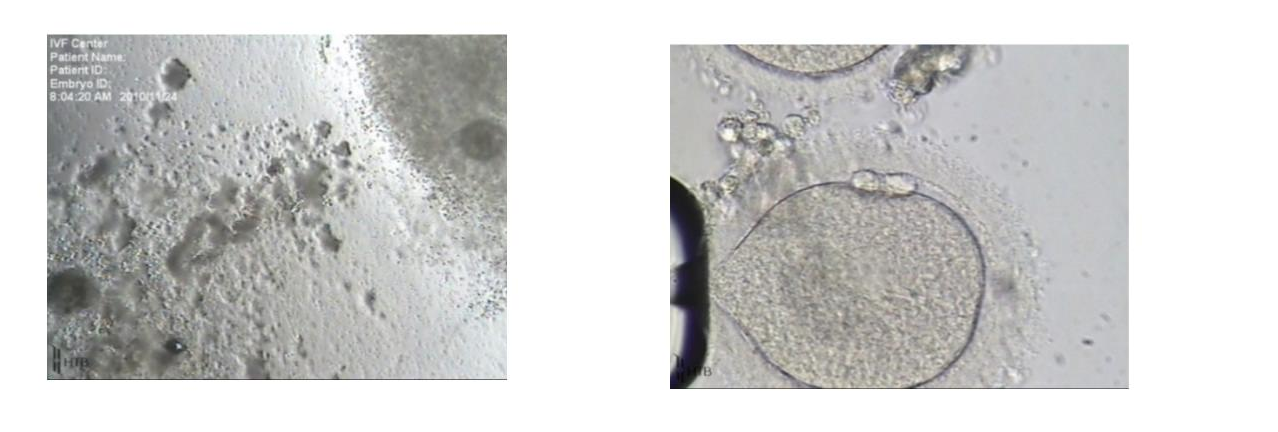
Figure 4. On the left, note the cumulus on the left has clumpy and degenerating appearance compared to the one on the right. On the right, a post-mature oocyte from the degenerating oocyte-cumulus complex on the left (Dozortsev, unpublished)
LH independent follicle rupture
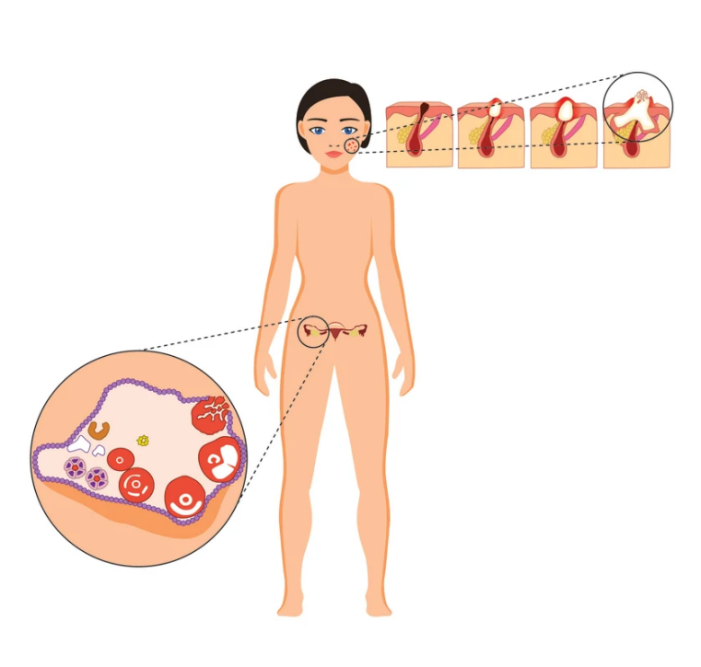
Figure 5. Similarity between ovarian and inflamed hair follicle
Both types of follicles are comparable histologically and in other key features:
· Begin at the similar distance under the surface
· Require GDF, BMP and SMAD
· Maximum size is determined by surrounding tissues
· Have a distinctive “ripe to rupture” stage
· Terminal phase of development involves prostaglandins
· Respond to steroids and NSAID
Ovarian follicle can rupture toward the end of the follicular phase without LH
The most relevant implication of similarity between the hair and ovarian follicle is that ovarian follicle does not need LH to rupture. It will rupture on its own as its continued expansion, even driven solely by FSH, can no longer be accommodated by the surrounding ovarian tissues. This has been convincingly demonstrated by Couzinet et all (1988) in patients with hypogonatrophic amenorrhea, lacking endogenous LH and in hypophysectomized rats (Hubbard and Erickson, 1988).
In IVF practice, this phenomenon is known as “vanishing follicles” and occurs more frequently in older patients during stimulated cycles. The vanishing of the follicle is accompanied by increase in circulating progesterone to the post-ovulation level, even when LH surge is suppressed (Kol, 2008).
It must be noted that strictly speaking, rupture of the follicle that has not been exposed to LH (or hCG) cannot be considered ovulation. Even though FSH can cause follicle rupture, it is not known to effect gap-junctions degeneration, which could result in trapping the egg inside of the ruptured follicle or ovulation of an immature egg or egg surrounded such tight cumulus that sperm penetration is not possible.
Role of LH
If the follicle’s rupture, resumption of meiosis and luteinization of granulosa cells can all take place in the absence of LH, what is accomplished by LH surge?
Even though ovulation events can each take place without LH, they have an extremely low probability to result in pregnancy without tight coordination in time, due to a relatively narrow viable fertilization window, which can be defined as fertilization that can result in term pregnancy (Dozortsev et al, 2004).
The precise duration of viable fertilization window in the human is unknown, however, the experience with day 1 ICSI clearly demonstrates that it is no longer 24 hrs post retrieval (Yuzpeand, 2000) or about 60 hrs post hCG.
Dozortsev et al (2003) demonstrated that the highest chance of an embryo developing to term is decreasing if fertilization took place later than 41 hours post hCG, even though the chance of fertilization continues to climb.
According to Dozortsev et al (2004) the oocyte has a highest chance to develop to term if it is fertilized between 37 and 41 hrs after hCG. If the sperm penetration of the oocyte took place earlier than 37 hrs post hCG, both fertilization rates and pregnancy drop.
This means that human oocyte has the highest chance for viable fertilization within only 4-5 hours after ovulation.
Since ovulation takes place 36-37 hrs after hCG, it is apparently tightly linked to the optimal fertilization window, which implies that putative messenger must witness follicles impending rupture and relate it to the GnRH to trigger LH surge.
Physiological LH surge is always accompanied by the surge in FSH and therefore is often referred to as LH/FSH surge.
It is not known whether the FSH is truly needed for the follicle rupture at this stage or simply a “side effect” of LH surge, which alone is sufficient to invoke both follicle rupture and ovulation. The possibility of FSH surge being a side-effect is created by colocation of FSH in the same cells and even in the same area of their cytoplasm (Bauer-Dantoin et al, 1993). The selectivity of their release is not determined by GnRH, but by other messengers, such as NPY protein (Bauer-Dantoin et al, 1993), providing a very plausible explanation for observing some amount of one with another (Figure 9).
Thus, the role of LH in ovulation is a coordination of resumption of meiosis and loosening of the cumulus cells with follicle’s rupture
Follicular phase and ovulation
Antral follicle development
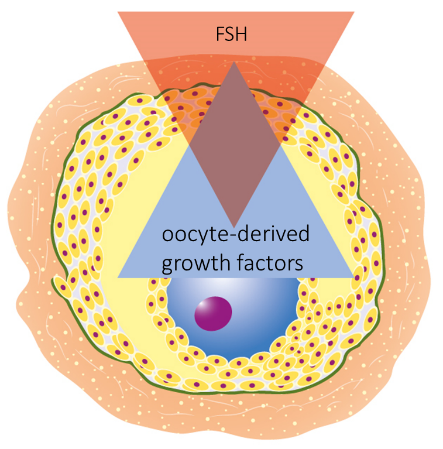
Figure 6. Oocyte and FSH competes for the control over granulosa cells
FSH becomes a second source of follicle’s control as oocyte is recruited into follicular phase
The development of the oocyte during the follicular phase will remain under control of the oocyte-produced growth factors. However, the control over the follicle enlargement, which is the result of mural granulosa proliferation and fluid accumulation, will be controlled by the FSH and later by both, FSH and LH, produced by the anterior pituitary.
Full recognition of this dual control is critical, because it helps to appreciate that the processes responsible for egg acquiring developmental competency and the process responsible for the ultimate egg release from the follicle (ovulation) are driven by completely different mechanisms, which are unaware of each other, even though they share place and time.
Competing relationship between FSH and oocyte-derived growth factors lead to “unfair” distribution of granulosa cells
As already stated, the commitment of the granulosa cells to acquiring FSH receptors is their intrinsic property and cannot be controlled externally.
However, the actual differentiation into cumulus and mural cells is a fluid process, since it is decided by competing gradients of the oocyte’s growth factors and FSH (Diaz, 2007).
The distribution of granulosa cells between oocyte and the follicle as a result of competition between oocyte and FSH is pivotal point in the oocyte competency, because it determines how many cells will continue nursing the egg and how many will be allocated to the follicular wall and produce estradiol (Figure 6).
All existing evidence point to the fact that high FSH levels will recruit more granulosa into mural, leaving fewer cells to remain nursing the egg (Eppig et al, 2002; Diaz et al, 2007).
It is not known how many cells should remain with the oocyte to adequately provide its support, but the lowest threshold probably exists. This creates a strong, albeit, theoretical possibility that excess of FSH at the recruitment phase may reduce the quality of the oocyte. Multiple studies demonstrating improvement of oocyte quality with mild ovarian stimulation support such assumption (Zhang et al, 2010).
Another unintended consequence of excessive recruitment of the granulosa into mural granulosa, even if the adequate number of cumulus cells is left to nurture the oocyte, is a disproportionally fast growing follicle, which may rupture prematurely.
Ovulation
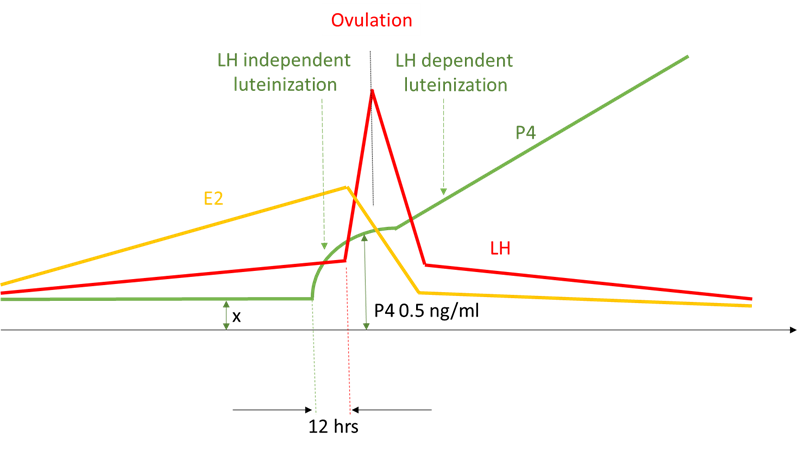
Figure 7. Ovulation dynamics extrapolated from Hoff et al, 1983
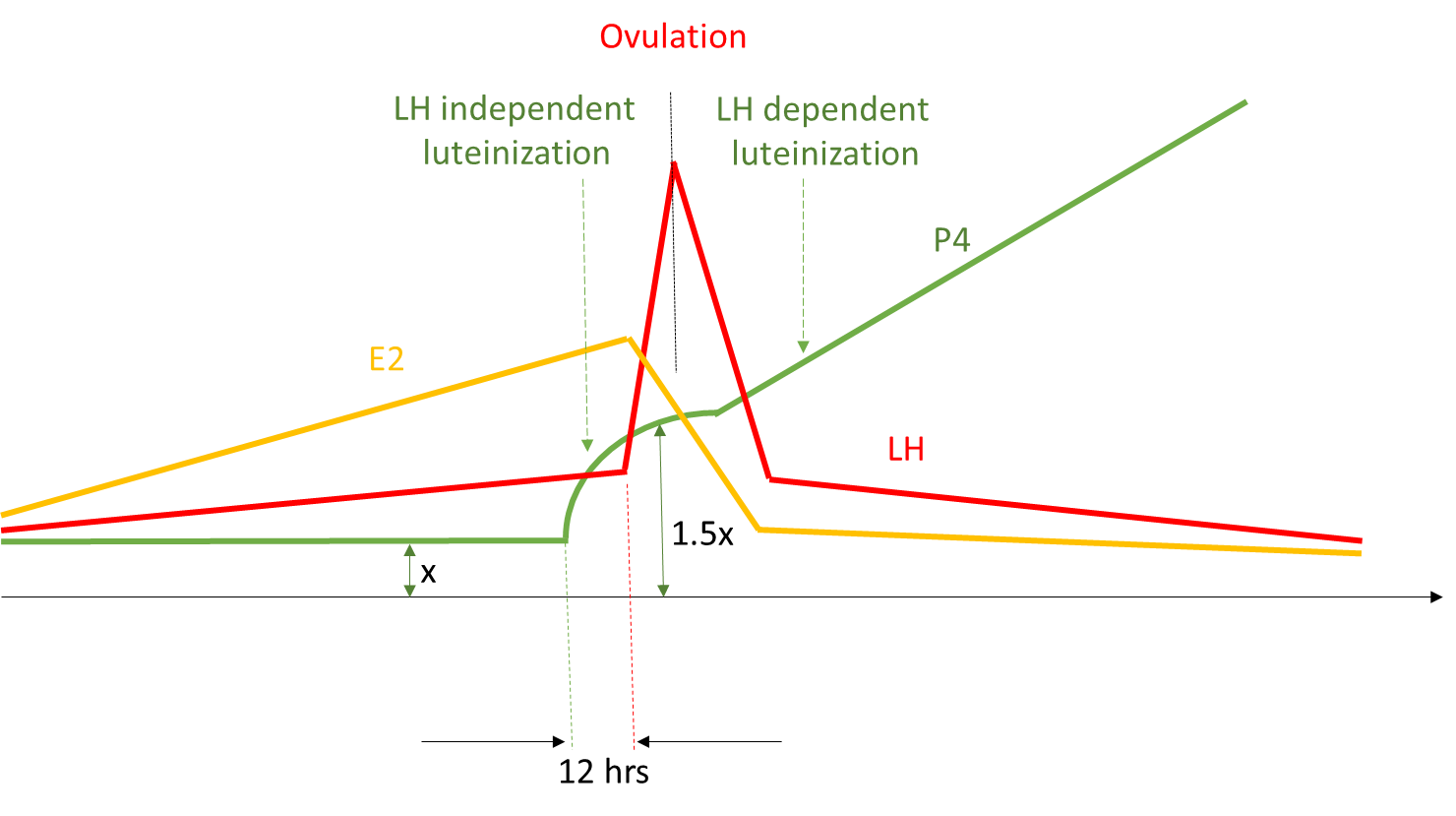
Figure 8. 1.5 times increase in circulating progesterone before LH surge (Dozortsev et, unpublished)
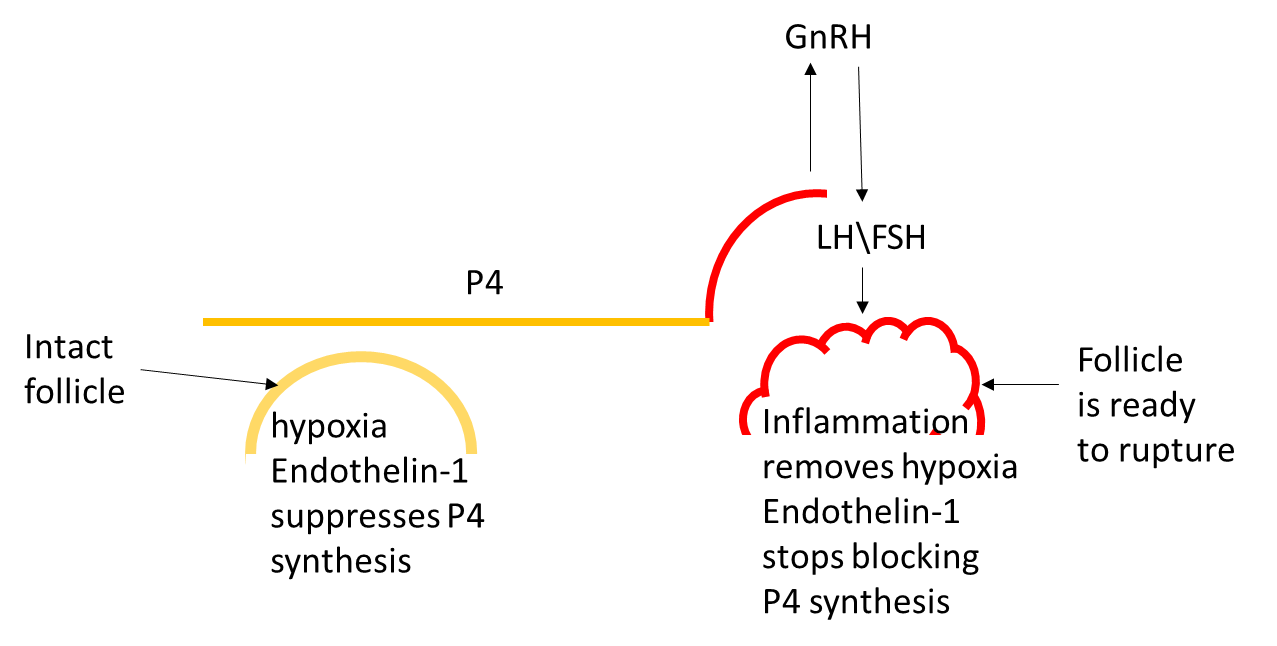
Figure 8. As the growing follicle reaches its maximum size (determined by the speed of the follicle growth, ovarian cortex elasticity and other factors), it inflames, removing hypoxia and blocking endothelin 1, which removes the block for progesterone synthesis, leading to precipitous rise of the progesterone in circulation.
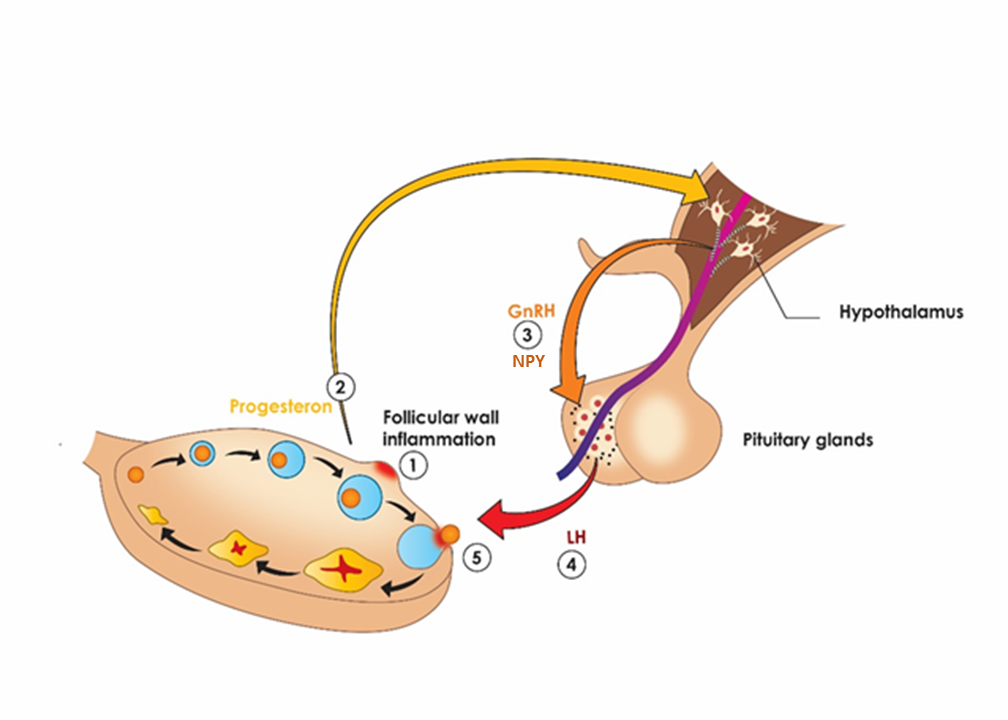
Figure 9. Proposed ovulation sequence: as the follicle begins to lose integrity (1) due to inflammation, some granulosa cells luteinize, leading to three -fold increase in circulating progesterone (2), which invokes the surge of GnRH and NPY (3). NPY selectively increases sensitivity of LH containing granins (Bauer-Dantoinet al, 1993) to GnRH resulting in preferential surge of LH (4), which causes the follicle to rupture and egg to ovulate (5)
It is also possible that several of the above mechanisms act in synergy, creating a redundancy.
Rapid release of LH and FSH boosts fluid accumulation in the follicle, which is already in the process of rapture, leads to the physical disruption of the gap-junctions between the oocyte and cumulus cells, disperses cumulus and sets into motion oocyte’s internal egg’s clock by leading to meiosis resumption within 30 min of LH surge so that it is released around 36-37 hrs just before the optimal viable fertilization window.
Resolving the contradictory actions of progesterone on ovulation.
Historically progesterone has been viewed as an ovulation blocking agent (Selye et al, 1936). This ability of progesterone is well known and is supported by vast experience with the variety of progestins in birth-control pills.
At the first sight, this is irreconcilable with the possibility of progesterone causing LH to surge and inducing an ovulation. Particularly, because the circulating level of progestins used in birth-control formulations is considerably smaller than the preovulatory level of progesterone of 0.5 ng/ml.
First crucial step in resolving this contradiction is understanding that ovulatory LH/FSH surge is the result of their accumulation within the respective granins during the preceding follicular phase. Without such accumulation, there is no surge.
Second, as we can see from the Table 1 the activity of nearly all progestins is considerably higher than that of progesterone. Therefore, comparing them to progesterone by amount in circulation is misleading. We need to compare them by their activity instead. The estimated activity of all progestins formulation in circulation is higher than an equivalent of 0.5 ng of preovulatory progesterone (Table 1).
| Progestin | Consensus value (mg) |
|---|---|
| Norethindrone | 0.35 |
| D/L norgestrel | 0.075 |
| MPA | 5 |
| Dydrogesterone | 10 |
Table 1. The relative amount of progestines to 200 mg progesterone to elicit changes equivalent to premenopausal, secretory phase (King and Whitehead, 1986)
Consequently, at those levels, progestins would be expected to both, desensitize progesterone or perhaps GnRH receptors and/or continuously drain pituitary of LH and FSH, interfering with their accumulation that is required for surge.
Thus, it can be inferred that when progesterone is very low, during the follicular phase, it allows LH to accumulate and receptors to become sensitized. As the result, when progesterone rises within a narrow window, of about 12 hrs preceding LH surge, it causes LH to flare-up and subsequent ovulation.
On the other hand, if progesterone activity is continuously present at the levels above its physiological pre-ovulatory level, such as the case with all birth control formulations, it causes desensitization of its own or GnRH receptors (McArdle et al, 1995), so that LH accumulation or its surge are not possible, and ovulation is blocked.
We believe that this is very plausible explanation, resolving the opposite actions of progesterone as an ovulation inducer and blocker.
Term oocyte maturation
Based on the information we have reviewed, the progesterone rises rapidly from a consistently suppressed level, as a direct result of imminent, impending rupture of the follicle, which makes it a better witness for the state of the follicle than estradiol, which rises continuously throughout follicular phase and has high variability rate from cycle to cycle.
Yet, importantly, as we have seen, just like estradiol, progesterone is not witnessing whether the egg has reached its developmental competency.
This indicates that evolutionary, rather than coupling the egg competency with ovulation directly, nature simply waits as long as tissues can accommodate the growing follicle’s expansion, giving the egg as much time as possible to mature.
This is consistent with epidemiological data that shorter menstrual cycle, which is almost always due to shortening of the shorter follicular phase, is associated with lower fecundity (Small et al, 2006; Lauren et al, 2011). At the same time, unusually long menstrual cycle that is due to the extended follicular phase, is not affecting fertility (Small et al, 2006; Lauren et al, 2011).
This could be interpreted as existence of a minim amount of time that is required for an egg to reach developmental competence during the follicular phase.
Since the follicle’s development during the follicular phase is divorced from the egg reaching its developmental competence, the time required for an egg to attain competence must be recognized as an independent variable, which we propose to refer to as Term Oocyte maturation (TOM).
Based on epidemiological data on the duration of the menstrual cycle, we believe that duration of TOM can be set at 14 days, starting from the first day of the period. Just like a term-pregnancy is used to define a safe time-limit for a fully developed child, TOM defines as a safe limit for a fully competent egg, which is capable of full development, once fertilized.
TOM is much more encompassing than merely ability to resume meiosis and become fertilized, which are attained considerably early.
Recognizing the existence of TOM, we do not suggest that every egg needs a minimum of 14 days to attain competency. Just like a child born at 8 months, can be completely healthy, yet considered pre-term.
We further believe that inability of the oocytes to attain TOM maybe responsible for a whole host of egg quality issues, the murkiest area of reproduction, in patients of all ages, both in the natural and stimulated cycles.
Ramifications of the hormone-independent luteinization induced LH surge in the human
Ramifications for controlled ovarian stimulation
One of the corner stones of the current view of ovulation is that the maturing oocyte communicates its state of readiness to the follicle, which will rupture once the egg has reached full maturity. In other words, the oocyte readiness is coupled with the follicle’s growth. Therefore, we use the size of the follicle and the level of hormones it produces as indirect markers of maturation of the corresponding oocyte. Accordingly, ovarian stimulation monitoring is structured to observe the follicle growth and hormones level, to render a decision about oocyte’s readiness for the development into the offspring, once fertilized and implanted into the uterus.
However, as we discussed, an oocyte contained within a tertiary follicle has no mechanism to communicate its developmental competence to the follicle and there is no coupling between oocyte developmental competence and follicle’s life-cycle. Therefore, the size of the follicle or the level of E2 cannot be considered as variables reflecting egg quality or developmental competence (Kupesicand Kurjak, 2002).
Another important ramification for controlled ovarian stimulation is that P4 surge during the stimulation is not a sign of inadequate LH suppression or activity of adrenal glands, but merely an indication that one of the follicles has ruptured prematurely. This obviously makes a fresh transfer not recommended but should not cause any concern about the quality of the remaining follicles or oocytes. You may also expect that some of the eggs will have post-mature appearance at the retrieval.
Ramifications for reproductive ageing
Decrease of the egg quality with age is a well-known phenomenon. It manifests as lower fecundity, which is based on indisputable epidemiological data, is due to the spike in the level of chromosomal errors in the oocytes.
One of the commonly accepted underlying reasons for the increased risk of chromosomal errors is oxidative damage of the DNA over time (Kefee et al, 2008).
However, it is also a common knowledge that as woman gets older, her menstrual cycle tends to shorten due to decrease duration of the follicular phase and they tend to ovulate with smaller dominant follicle (Santoro et al, 2003).
We agree with Kol (2008) that the loss of ovarian elasticity, which causes premature follicle rupture is a highly plausible explanation for this observation. Even if it is not entirely correct and there are some other mechanisms leading to ovarian tissue inability to accommodate full expansion of the follicle, the shortening of the follicular phase itself leaves less time for the egg to reach its full developmental potential.
This problem in older patients is further exacerbated by reduced ovarian reserve, which leads to higher FSH levels at the start of the follicular phase, which in its own turn causes the follicle to initially grow faster (Santoro et al, 2003), making it even harder for the cortex to accommodate the expansion. This extra FSH at the onset of the follicular phase, would also tend to shift the balance of granulosa cells away from the oocyte to mural granulosa.
Therefore, we believe that it is possible that age-related eggs abnormalities, including chromosomal abnormalities, are due in part to inability of oocyte to reach TOM due to ovarian tissues ageing.
We speculate that the decrease of oocyte quality with age, including the increase in the percentage of chromosomally abnormal oocytes may well be due to depriving the oocyte of reaching TOM due to accelerated pace of follicular expansion combined with the diminished ability of the ovarian tissues to accommodate the expansion.
That would explain why minimal stimulation, which increases the duration of the follicular phase, yields better outcomes in this group of patients.
This hypothesis can be easily testes in women of advanced reproductive age, by artificially extending the duration of the follicular phase.
Since we do not need to worry about the size of the follicle, but only about the time, this may be achieved, for example, by administering small amount of estrogens starting from the last few days of the luteal phase to lower the initial FSH and then Diclofenac and Lupron to prevent premature rupture. That would be expected to extend the follicular phase and improve the oocyte quality and perhaps decrease the rate of chromosomal error in oocytes.
Ramifications for a search of more natural ovulation trigger
Triggering ovulation is a crucial step in the management of the controlled ovarian stimulation in patients undergoing IVF, IUI, timed intercourse and other forms of fertility therapy. The ovulation trigger not only ultimately responsible for the last stages of oocyte maturation and follicle’s rupture but also primes the endometrium for subsequent implantation.
Currently hCG is the only medication specifically approved by FDA as an ovulation trigger. However, its use is rapidly declining due to the relatively high incidence of the ovarian hyperstimulation syndrome (OHHS). Lupron Acetate is increasingly used an off-label ovulation drug of choice due to its low incidence of OHHS and a generally good record of safety and efficiency. Yet, Lupron is expensive and has a several unpleasant side effects.
Several derivatives of Kisspeptin are in the process of investigation (Phase II), but are expected to be on the expensive side, should they reach the market (Abbara et al, 2017).
The important shortcoming of all currently available triggers, including Kisspeptin, is there inability to fully reproduce the naturally occurring pulsating pattern of GnRH release, which is believed to be a consequential feature of the process.
In order to compensate for this deficiency a Kisspeptin, for example, has to be injected several times. Also, in order to mimic the pulsating nature of GnRH release, a successful use of the pump has been reported (Pulsatile GnRH). Neither is clinically practical for a general infertility population.
If progesterone is indeed a most upstream ovulation trigger, it could become a very inexpensive drug of choice, which would mimic natural ovulation.
Conclusion
There is a number of experimental and clinical evidence pointing to the preovulatory, LH-independent progesterone rise as a trigger for ovulatory gonadotropins flare. However, the shift of the paradigm has been slow, as often is a case in medicine and biology.
We hope that this review will help to engage a larger audience in more open-minded look at the role of progesterone in ovulation.
References:
Abbara A, Clarke S, Islam R, Prague JK, Comninos AN, Narayanaswamy S, Papadopoulou D, Roberts R, Izzi-Engbeaya C, Ratnasabapathy R, Nesbitt A, Vimalesvaran S, Salim R, Lavery SA, Bloom SR, Huson L, Trew GH, Dhillo WS. A second dose of kisspeptin-54 improves oocyte maturation in women at high risk of ovarian hyperstimulation syndrome: a Phase 2 randomized controlled trial. Hum Reprod. 2017 Sep 1;32(9):1915-1924.
Batista MC1, Cartledge TP, Zellmer AW, Nieman LK, Merriam GR, Loriaux DL. Evidence for a critical role of progesterone in the regulation of the midcycle gonadotropin surge and ovulation. J Clin Endocrinol Metab. 1992 74(3):565-70.
Bauer-Dantoin AC1, Tabesh B, Norgle JR, Levine JE. RU486 administration blocks neuropeptide Y potentiation of luteinizing hormone (LH)-releasing hormone-induced LH surges in proestrous rats. Endocrinology. 1993 Dec;133(6):2418-23.
Bryce RL, Shuter B, Sinosich MJ, Stiel JN, Picker RH, Saunders DM. The value of ultrasound, gonadotropin, and estradiol measurements for precise ovulation prediction. Fert and Ster, 1982 37(1):42-45
Buchholz r, Nocke l, Nocke w. The influence of gestagens on the urinary excretion of pituitary gonadotropins, estrogens, and pregnanediol in women in the postmenopause and during the menstrual cycle. Int J Fertil, 1964 Jan-Mar;9:231-51
Christensen A, Bentley GE, Cabrera R, Ortega HH, Perfito N, Wu TJ, Micevych P. Hormonal regulation of female reproduction. HormMetab Res. 2012 Jul;44(8):587-91. doi: 10.1055/s-0032-1306301. Epub 2012 Mar 21.
Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001 Jun 20;179(1-2):97-103.
Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007 Apr 15;120(Pt 8):1330-40. Epub 2007 Mar 27. Dozortsev D, Nagy P, Abdelmassih S, Oliveira F, Brasil A, Abdelmassih V, Diamond M, Abdelmassih R. The optimal time for intracytoplasmic sperm injection in the human is from 37 to 41 hours after administration of human chorionic gonadotropin. Fertil Steril. 2004 Dec;82(6):1492-6.
Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):2890-4. Epub 2002 Feb 26.
Gilchrist RB1, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008 Mar-Apr;14(2):159-77.
Goudet G1, Bézard J, Duchamp G, Gérard N, Palmer E, Equine oocyte competence for nuclear and cytoplasmic in vitro maturation: effect of follicle size and hormonal environment. Biol Reprod. 1997 Aug;57(2):232-45
Hoff JD, Quigley ME and Yen SSC, Hormonal Dynamics at Midcycle: A Reevaluation. The Journal of Clinical Endocrinology & Metabolism, 1983 57 (4):792–796
Hubbard GM1, Erickson GF. Luteinizing hormone-independent luteinization and ovulation in the hypophysectomized rat: a possible role for the oocyte? Biol Reprod. 1988 Aug;39(1):183-94.
Hurst BS*, Merriam KS , Elliot M, Matthews ML, Marshburn PB, and Rebecca S Usadi RS . A Sustained Elevated Estradiol is not the Trigger for the Preovulatory Luteinizing Hormone Surge, Women’s Health & Gynecology, 2015 1(2):1-3
JayasenaCN. ,Trew GH. , Waljit S. Dhillo WS. Kisspeptin-54 triggers egg maturation in women undergoing in vitro fertilization, J Clin Invest. 2014 124(8):3667-3677
King RJ and Whitehead MI. Assessment of the potency of orally administered progestins in women. FertilSteril. 1986 46(6):1062-6.
Keefe DL, Marquard K, Liu L. The telomere theory of reproductive senescence in women. Curr Opin Obstet Gynecol. 2006 Jun;18(3):280-5.
Klein NA. , Harper AJ. , Houmard BS. , Sluss PM., SoulesIs MR. the Short Follicular Phase in Older Women Secondary to Advanced or Accelerated Dominant Follicle Development? Impaired Folliculogenesis and Ovulation in Older Reproductive Aged Women
Kol S. The vanishing follicle in women aged over forty: premature, mechanical, LH-independent luteinization may reflect oocyte-follicle low quality? Med Hypotheses. 2008;70(6):1227-8
King RJ, Whitehead MI. Assessment of the potency of orally administered progestins in women. Fertil Steril. 1986 Dec;46(6):1062-6.
Kubiak JZ1. Mouse oocytes gradually develop the capacity for activation during the metaphase II arrest. Dev Biol. 1989 Dec;136(2):537-45.
Kupesic S1, Kurjak A. Predictors of IVF outcome by three-dimensional ultrasound. Hum Reprod. 2002 Apr;17(4):950-5.
Lauren A. Wise,* Ellen M. Mikkelsen, Kenneth J. Rothman, Anders H. Riis, Henrik ToftSørensen, Krista F. Huybrechts, and Elizabeth E. Hatch A Prospective Cohort Study of Menstrual Characteristics and Time to Pregnancy. Am J Epidemiol. 2011 Sep 15; 174(6): 701–709.
Leavitt WW, Chen TJ, Allen TC. Regulation of progesterone receptor formation by estrogen action. Ann N Y Acad Sci. 1977 Mar 11;286:210-25.
Liu JH, Yen SS. Induction of midcycle gonadotropin surge by ovarian steroids in women: a critical evaluation. J Clin Endocrinol Metab. 1983 Oct;57(4):797-802.
Lydon JP1, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995 Sep 15;9(18):2266-78.
McArdle CA, Forrest-Owen W, Willars G, Davidson J, Poch A, Kratzmeier Desensitization of gonadotropin-releasing hormone action in the gonadotrope-derived alpha T3-1 cell line. M. Endocrinology. 1995 Nov;136(11):4864-71.
Murphy BD. Models of luteinization. Biol Reprod. 2000 Jul;63(1):2-11.
Nanette Santoro, Barbara Isaac, Genevieve Neal-Perry, Tovaghol Adel, Laura Weingart,Aimee Nussbaum, Shilpa Thakur, Hikoyoshi Jinnai, Nidhi Khosla, David Barad The Journal of Clinical Endocrinology & Metabolism, Volume 88, Issue 11, 1 November 2003, Pages 5502–5509
Odell WD and Swerdloff RS, Progestogen-induced luteinizing and follicle-stimulating hormone surge in postmenopausal women: a simulated ovulatory peak. PNAS 1968 61(2):529-536
Rosenfield RL1, Lucky AW. Acne, hirsutism, and alopecia in adolescent girls. Clinical expressions of androgen excess. Endocrinol Metab Clin North Am. 1993 Sep;22(3):507-32.
Rothchild I. Interrelations between progesterone and the ovary, pituitary, and central nervous system in the control of ovulation and the regulation of progesterone secretion. VitamHorm. 1965 23:210-327.
Sanfins A1,2, Rodrigues P3, Albertini DF4. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet. 2018 Oct;35(10):1741-1750. doi: 10.1007/s10815-018-1268-4. Epub 2018 Jul 23.
Santoro N1, Isaac B, Neal-Perry G, Adel T, Weingart L, Nussbaum A, Thakur S, Jinnai H, Khosla N, Barad D. Impaired folliculogenesis and ovulation in older reproductive aged women.J Clin Endocrinol Metab. 2003 Nov;88(11):5502-9.
H. Selye, J. S. L. Browne, J. B. Collip Effect of Large Doses of Progesterone in the Female Rat. Proceedings of the Society for Experimental Biology and Medicine, Volume: 34 issue: 4, page(s): 472-474, 1936
Smith G1, Roberts R, Hall C, Nuki G. Reversible ovulatory failure associated with the development of luteinized unruptured follicles in women with inflammatory arthritis taking non-steroidal anti-inflammatory drugs. Br J Rheumatol. 1996 May;35(5):458-62.
Stout EP, La Clair JJ, Snell TW, Shearer TL, Kubanek J. Conservation of progesterone hormone function in invertebrate reproduction Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11859-64. doi: 10.1073/pnas.1006074107. Epub 2010 Jun 14.
Takahashi T, Ohnishi J. Molecular mechanism of follicle rupture during ovulation. Zoolog Sci. 1995 Aug;12(4):359-65.
Tedeschi C, Hazum E, Kokia E, Ricciarelli E, Adashi EY, Payne DW. Endothelin-1 as a luteinization inhibitor: inhibition of rat granulosa cell progesterone accumulation via selective modulation of key steroidogenic steps affecting both progesterone formation and degradation. Endocrinology. 1992 Nov;131(5):2476-8.
Tedeschi C, Hazum E, Kokia E, Ricciarelli E, Adashi EY, Payne DW. Endothelin-1 as a luteinization inhibitor: inhibition of rat granulosa cell progesterone accumulation via selective modulation of key steroidogenic steps affecting both progesterone formation and degradation. Endocrinology. 1992 Nov;131(5):2476-8.
Testart J, Thebault A, Souderes E, Frydman R. Toward the end of the follicular cycle the follicle become fragile and can easily rupture even by such low impact intervention as a follicular ultrasound. Premature ovulation after ovarian ultrasonography. Br J ObstetGynaecol. 1982 Sep;89(9):694-700.
Channing CP, Schaerf FW, Anderson LD, Tsafriri A.Ovarian follicular and luteal physiology. Int Rev Physiol. 1980;22:117-201. Review.
Wehrenberg U, Rune GM. Spontaneous luteinization of antral marmoset follicles in vitro. Mol Hum Reprod. 2000 Jun;6(6):504-9.
White MM, Sheffer I, Teeter J, Apostolakis EM. Hypothalamic progesterone receptor-A mediates gonadotropin surges, self priming and receptivity in estrogen-primed female mice. J Mol Endocrinol. 2007 Feb;38(1-2):35-50.
Yuzpe AA1, Liu Z, Fluker MR. Rescue intracytoplasmic sperm injection (ICSI)-salvaging in vitro fertilization (IVF) cycles after total or near-total fertilization failure. FertilSteril. 2000 Jun;73(6):1115-9.
Zalányi, S. (2001). Progesterone and ovulation. European Journal of Obstetrics & Gynecology and Reproductive Biology, 98(2), 152–159
Zhang J1, Chang L, Sone Y, Silber S. Minimal ovarian stimulation (mini-IVF) for IVF utilizing vitrification and cryopreserved embryotransfer. Reprod Biomed Online. 2010 Oct;21(4):485-95. doi: 10.1016/j.rbmo.2010.06.033. Epub 2010 Jun 30.
Santoro N1, Isaac B, Neal-Perry G, Adel T, Weingart L, Nussbaum A, Thakur S, Jinnai H, Khosla N, Barad D. Impaired folliculogenesis and ovulation in older reproductive aged women.J Clin Endocrinol Metab. 2003 Nov;88(11):5502-9.
Small CM, Manatunga AK, Klein M, Feigelson HS, Dominguez CE, McChesney R, Marcus M. Menstrual cycle characteristics: associations with fertility and spontaneous abortion.Epidemiology. 2006 Jan;17(1):52-60.
авапвап
вапвапвап
вапвапвап
вапвапвап
вапвапвап
вапвапвап
впавапвап
